Manufacturing medical devices is complex.
But it's all we do.
End-to-end contract manufacturing of medical devices & their components.
We manufacture success.
We scale production of our clients’ medical devices and components with consistent quality, process and regulatory compliance. From securing the supply chain to final assembly, QA and packaging, our clients entrust us to take care of every detail.
Ready to solve your Medical Device production challenges?
END-TO-END THINKING
Providing a single partner solution
We start by understanding your design, and offer Design for Manufacturing & Assembly expertise. We source materials and negotiate with trusted suppliers for a cost-efficient and reliable supply chain. We provide final assembly, typically fabricating all or most of the product’s components, with automated and hybrid solutions; all while meeting quality and regulatory standards, including Clean Room and sterilization capabilities. Moreover, we provide complete labeling, lot tracking, and final packaging.
Our end-to-end approach allows our clients to focus on their business and trust the production details to us.
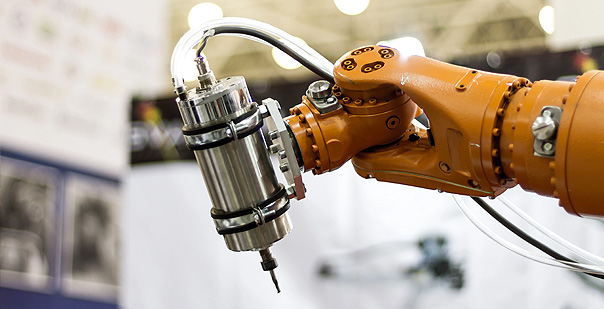
Assembly & Fabrication
The right tools for the job
We offer a broad range of assembly & manufacturing capabilities, from Clean Room assembly to volume automation; from complex digital devices to single-use, disposable products.
Quality & Certification
Quality is a foundation for trust

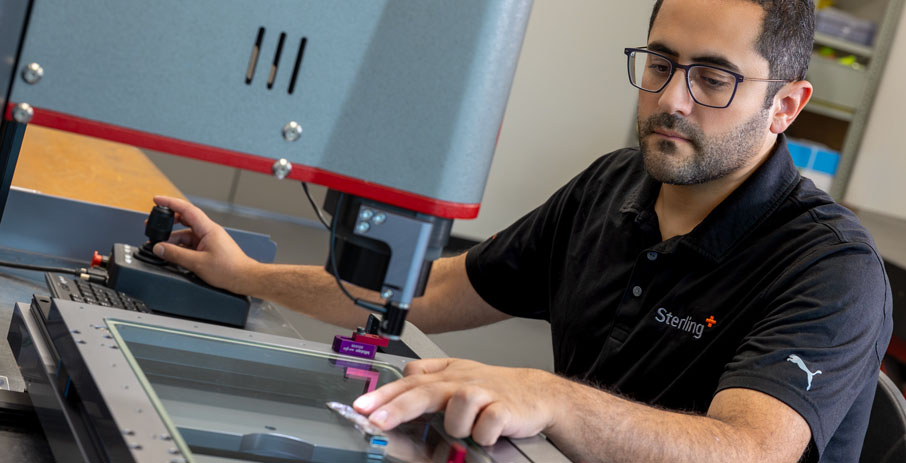
Process & Design
Your design, refined
From manufacturing transfers to Design for Manufacturing (DFM) to Continuous Improvement, we help our clients scale their products efficiently.
Commitment
Making success simple
Why Choose Us
Why Sterling?
We specialize.
We manufacture success.
We are experienced.
We are here.
We are right-sized.
We are well-priced.
With expertise in Design for Manufacturing (DFM) and sourcing, we optimize cost without sacrificing quality.









